Abstract
Introduction: Approximately 30% of patients with venous thromboembolism (VTE) experience a recurrence within 10 years of the initial event with their recurrence risk peaking during the first 6-12 months. Two large randomized clinical trials AMPLIFY-EXT and PADIS-PE reported that extended treatment with apixaban and warfarin beyond 6 months of initial treatment reduced recurrent VTE without increasing the rate of major bleeding compared to placebo, respectively. Little is known about real-world effectiveness and safety of extended oral anticoagulation beyond 6 months of initial treatment for Medicare beneficiaries with VTE, despite the fact that VTE disproportionately affects the elderly. We assessed the effectiveness and safety of extended use of apixaban and warfarin beyond 6 months of initial treatment for prevention of recurrent VTE and adverse major bleeding events among Medicare beneficiaries with newly diagnosed VTE.
Methods: A retrospective cohort study using 2014-2018 Medicare data (5% samples in 2014-2016 and 15% samples of Medicare beneficiaries in 2017-2018) was conducted for patients aged ≥18 years with a diagnosis of deep vein thrombosis or pulmonary embolism ascertained from inpatient claims. Patients were included if they initiated anticoagulants within 30 days of their first VTE diagnosis, completed 6 months of therapy defined as ≥83% proportion days covered with oral anticoagulants during the initial 6-month period, and received extended treatment with either apixaban or warfarin or no extended therapy. We compared the risks of recurrent VTE and major bleeding between apixaban, warfarin, and no treatment groups. To adjust for differences in baseline characteristics and clinical factors (e.g., HAS-BLED score, active cancer, and provoked VTE) between groups, we used the stabilized inverse probability treatment weighting (IPTW) method. Follow-up continued until the occurrence of the first event, switch to the comparator, disenrollment, death, or end of the study period. Multivariable Cox proportional hazards modeling with IPTW was used to obtain adjusted hazard ratios (aHR) and 95% confidence intervals (95%CI).
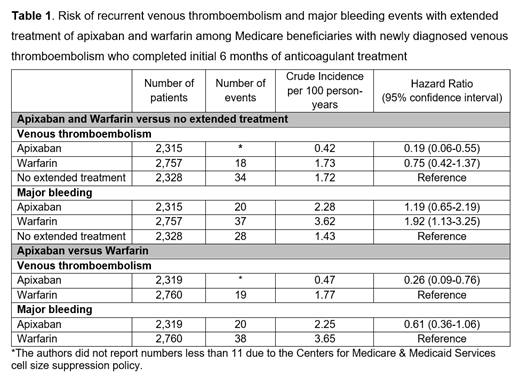
Results: The study cohort (mean age=74 ±12 years, 40% male, 76% White) consisted of 2,315 users of extended apixaban treatment (83% with 5 mg twice a day and 17% with 2.5 mg twice a day; mean duration=6.2 months), 2,757 users of extended warfarin treatment (mean duration=8.2 months), and 2,328 patients with no extended treatment following completion of an initial 6 months of anticoagulant treatment. The incidence rates of recurrent VTE were 0.42, 1.73, and 1.72 per 100 person-years, and those of major bleeding were 2.28, 3.62, and 1.43 per 100 person-years in the apixaban, warfarin, and no treatment groups, respectively (Table 1). Compared to no extended treatment, the use of apixaban was associated with an 80% decreased risk of recurrent VTE (aHR=0.19, 95%CI=0.06-0.55) without increasing the risk of major bleeding (aHR=1.19, 95%CI=0.65-2.19); the use of warfarin did not lower the risk of recurrent VTE (aHR=0.75, 95%CI=0.42-1.37) but increased the risk of major bleeding (aHR=1.92, 95%CI=1.13-3.25). Compared to the use of warfarin, the use of apixaban was associated with a decreased risk of recurrent VTE (aHR=0.26, 95% CI=0.09-0.76) and no difference in major bleeding risk (aHR=0.61, 95%CI=0.36-1.06). These findings remained consistent in subgroup (e.g., patients with provoked vs. unprovoked VTE, patients with active cancer vs. those without, and patients with chronic kidney diseases vs. those without) and sensitivity analyses (e.g., ≥92% proportion days covered with oral anticoagulants during the initial 6-month period).
Conclusions: Compared to no extended therapy, extended anticoagulation with apixaban was associated with a reduced risk of recurrent VTE without increasing the risk of major bleeding, whereas warfarin did not lower risk of recurrent VTE but increased the risk of major bleeding among Medicare beneficiaries with VTE. In the head-to-head comparison, the use of apixaban was more effective than warfarin in preventing recurrent VTE, without increasing the risk of major bleeding events. Our findings suggest that apixaban is an effective and safer option for extended treatment of VTE when compared to warfarin or no treatment among Medicare beneficiaries with VTE.
Park: BMS/Pfizer Alliance American Thrombosis Investigator Initiated Research Program: Research Funding. Kang: BMS/Pfizer Alliance American Thrombosis InvestigatorInitiated Research Program: Research Funding. Huang: BMS/Pfizer Alliance American Thrombosis Investigator Initiated Research Program: Research Funding. Lo-Ciganic: MERCK: Research Funding; BMS/Pfizer Alliance American Thrombosis Investigator Initiated Research Program: Research Funding. Dietrich: BMS/Pfizer Alliance American Thrombosis Investigator Initiated Research Program: Research Funding. Murphy: North American Thrombosis Foundation: Honoraria. DeRemer: BMS/Pfizer Alliance American Thrombosis Investigator Initiated Research Program: Research Funding; Portola Pharmaceuticals: Current equity holder in publicly-traded company; BMS advisory board attendee: Honoraria.
Author notes
 This icon denotes a clinically relevant abstract
This icon denotes a clinically relevant abstract

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal